Introduction: Hypomethylating agents (HMA) with Venetoclax (Ven) is the standard of care for patients (pts) with acute myeloid leukemia (AML) ≥ 75 yrs of age or not candidates to intensive treatment. Döhner, et al. (ASH 2022) demonstrated that the European LeukemiaNet (ELN) risk classifications do not accurately predict prognosis in HMA-Ven treated pts. A prognostic risk signature classification (PRSc) was proposed, stratifying pts by RAS, FLT3 and TP53 mutations alone. This classification has not been validated in low intensity treatments combining Ven with cladribine (CLAD) and cytarabine. Moreover, RAS mutations are common mechanisms of resistance to HMA-Ven, whilst it has been proposed that cytarabine and recently cladribine-based regimens could be effective in AML with this mutation. We report an update of pts treated with CLAD, low dose cytarabine (LDAC) and Ven, focusing on outcomes and risk group stratification.
Methods: This analysis included pts with newly diagnosed AML enrolled in a phase 2 clinical trial with CLAD-LDAC-Ven induction therapy followed by a consolidation phase that alternates courses of azacytidine and Ven with courses of CLAD-LDAC-Ven (NCT03586609). The PRSc allocated pts with [ K/N]- RAS or FLT3-ITD mutations in the intermediate benefit group, pts with TP53 in the lower benefit group, whereas pts with AML lacking these mutations were allocated in the higher benefit group. Cumulative incidence (CI) was calculated for pts achieving remission with death and relapse as competing events.
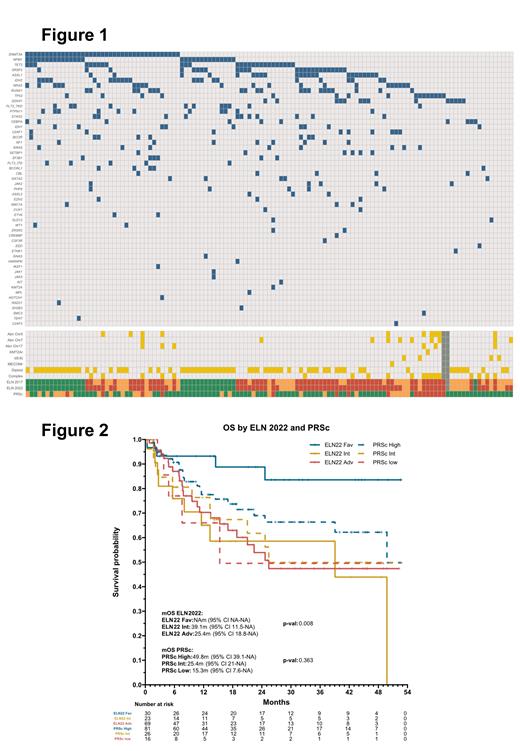
Results: From 11/2018 to 04/2023, 123 pts were treated on the CLAD-LDAC-Ven clinical trial. Median age was 68 yrs (range, 47-84) and 57% were male. 54% of pts had diploid cytogenetics and 16% had complex karyotype. Most frequent mutations were DNMT3A (32%), NPM1 (24%), TET2 (22%), SRSF2 (20%) and ASXL1 (19%). According to the ELN 2022 classification, 31 (25%), 22 (18%) and 69 (57%) were allocated in the favorable, intermediate and adverse risk categories, respectively. According to the PRSc, 81 (66%), 26 (21%) and 16 (13%) pts were allocated in the higher, intermediate and lower benefit group, respectively (Figure 1).
The overall response rate (ORR) was 85% (105/123), including 92 pts (75%) with complete remission (CR) rate and 13 (10%) having CR with incomplete hematologic recovery (CRi). Among pts achieving response, 78% (82/105) achieved MRD negativity by flow cytometry. According to ELN 2022 classification, ORR was 97%, 82% and 81% for favorable, intermediate, and adverse risk pts, respectively. According to the PRSc, the ORR was 89%, 85% and 69% for pts allocated in the higher, intermediate and lower benefit group. The median number of treatment courses received was 2 (1-18) and 94% of pts achieved best response after first cycle. After achieving remission, 48 (39%) pts underwent allogeneic stem cell transplantation (alloSCT).
The median OS and EFS were 50 months, and the 2-year cumulative incidence of relapse and death without relapse were 22% and 16%, respectively. The 1- and 2-year OS was 76% and 65%, respectively. According to the ELN 2022 classification, median OS was not reached, 39, and 25 months for favorable, intermediate and adverse risk groups (p=0.004, C-index=0.61). According to the PRSc, the median OS was 50, 25 and 15 months for the higher, intermediate and lower benefit group, respectively (p=0.36, C-index=0.56). Median EFS was 53, 15 and 19 months for ELN 2022 favorable, intermediate and adverse risk, respectively. According to the PRSc, the median EFS was 52, 25 and 17 months for months for the higher, intermediate and lower benefit group, respectively (Figure 2).
The 2-yr CI of relapse was 13%, 18% and 28% for pts of allocated in the ELN favorable, intermediate and adverse group, respectively. When using the PRSc classification, the 2-yr CI of relapse was 18%, 17% and 51% for the higher, intermediate and lower benefit group, respectively.
Conclusion: CLAD-LDAC-Ven combination offers an encouraging ORR with long term OS and EFS. In these pts, the ELN 2022 showed a better risk group discrimination regarding OS, compared to the PRSc. Pts of the intermediate-benefit of the PRSc (enriched in RAS/FLT3 mutations) could benefit the most of CLAD-LDAC-Ven, in which an HMA-Ven approach is associated with a mOS of 12 months.
Disclosures
Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees. Short:Amgen: Honoraria; Takeda: Consultancy, Research Funding; AstraZeneca: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Astellas: Research Funding; Stemline therapeutics: Research Funding. Jabbour:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. Daver:Syndax: Consultancy; Trovagene: Research Funding; FATE: Research Funding; Genentech: Consultancy, Research Funding; Novimmune: Research Funding; Jazz: Consultancy; Hanmi: Research Funding; AbbVie: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; AROG: Consultancy; Glycomimetics: Research Funding; Agios: Consultancy; Shattuck Labs: Consultancy; Amgen: Consultancy, Research Funding; Novartis: Consultancy; Servier: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Kite, a Gilead company: Consultancy, Research Funding; Kronos Bio: Research Funding. Issa:Novartis: Consultancy, Research Funding; NuProbe: Consultancy; Syndax: Research Funding; Celgene: Research Funding; Kura Oncology: Consultancy, Research Funding; Merck: Research Funding. DiNardo:BMS: Honoraria; AbbVie/Genentech: Honoraria; Servier: Honoraria; ImmuniOnc: Honoraria; Notable Labs: Honoraria; Fogham: Honoraria; Schrödinger: Consultancy; Takeda: Honoraria; Novartis: Honoraria; Astellas: Honoraria. Pemmaraju:Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karger Publishers: Other: Licenses; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; United States Department of Defense (DOD): Research Funding; ASCO Cancer.Net Editorial Board: Other: Leadership; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Jain:Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pfizer: Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; ADC Therapeutics: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Takeda: Research Funding; Mingsight: Research Funding; Fate Therapeutics: Research Funding; MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Incyte: Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Aprea Therapeutics: Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Medisix: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Newave: Research Funding; Dialectic Therapeutics: Research Funding; TransThera Sciences: Research Funding; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Novalgen: Research Funding; Loxo Oncology: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Servier: Research Funding. Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding. Wierda:Loxo Oncology, Inc./Lilly: Research Funding; Juno Therapeutics: Research Funding; Janssens Biotech Inc: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; GlaxoSmithKline: Research Funding; Cyclacel: Consultancy, Research Funding; Miragen: Research Funding; Janssens Biotech: Research Funding; GSK/Novartis: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Numab THerapeutics: Research Funding; Nurix THerapeutics: Research Funding; Accutar Biotechnology: Research Funding; Sunesis: Research Funding; KITE Pharma: Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; Gilead Sciences: Research Funding; AstraZeneca/Acerta Pharma: Consultancy, Research Funding; Pharmacyclics LLC: Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Research Funding. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Ravandi:Biomea fusion: Honoraria, Research Funding; Xencor: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Amgen: Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kantarjian:AstraZeneca/MedImmune: Honoraria; Abbvie: Consultancy, Honoraria; Ipsen: Honoraria; Immunogen (Inst): Honoraria, Research Funding; Daiichih-Sankyo (Inst): Honoraria, Research Funding; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; KAHR Medical: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Precision Biosciences: Honoraria; Shenzhen Target Rx: Honoraria; Taiho Pharmaceutical: Honoraria; Abbvie (Inst): Research Funding; Amgen (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; Novartis (Inst): Research Funding; Astellas Pharma: Honoraria; Ascentage Pharma Group: Honoraria; Amgen: Honoraria. Kadia:Genzyme: Honoraria; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Amgen, Inc.: Research Funding; Iterion: Research Funding; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Janssen Research and Development: Research Funding; Genentech: Consultancy, Research Funding; Cure: Speakers Bureau; Cyclacel: Research Funding; Ascentage Pharma Group: Research Funding; Cellenkos Inc.: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; GenFleet Therapeutics: Research Funding; Glycomimetics: Research Funding; Astellas Pharma Global Development: Research Funding; SELLAS Life Sciences Group: Research Funding; Sanofi-Aventis: Consultancy; AstraZeneca: Research Funding; Celgene: Research Funding; Regeneron Pharmaceuticals: Research Funding; Liberum: Consultancy; Hikma Pharmaceuticals: Speakers Bureau; Delta-Fly Pharma, Inc.: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal